Fluorine-containing 2,3-diaryl quinolines as potent inhibitors of methicillin and vancomycin-resistant Staphylococcus aureus: Synthesis, antibacterial activity and molecular docking studies
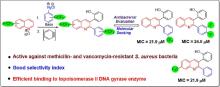
| Abstract | Drug resistant bacteria pose a major health concern and affect a large section of global population. Antibacterial drug discovery has stagnated owing to multiple factors including unattractive returns for major pharmaceutical companies. Thus, discovery of effective antibacterial drugs against drug-resistant bacteria is an urgent unmet need affecting healthcare systems globally. In this study, fluorine-containing 2,3-diarylquinolines (4a-l) and non-fluorinated analog 4m were synthesized utilizing environmentally benign chemistry of arenediazonium salts and arynes for regioselective installation of aryl groups at C-2 and C-3 positions, respectively. In vitro antibacterial evaluation against various Gram-negative and Gram-positive bacteria revealed inhibitory activity of majority of these compounds against Gram-positive S. aureus ATCC 29213. Compounds 4e, 4i, 4j and 4l were most potent inhibitors with MIC values of 10.95−24.0 µM. None of the compounds inhibited Gram-negative bacteria. 4e, 4i and 4l also displayed low levels of cytotoxicity against Vero cells, therefore, offering high safety profiles. Importantly, 4e, 4i and 4l exhibited equipotent inhibition of Methicillin and Vancomycin-resistant S. aureus, rendering them potential hits for further development. Molecular docking studies with topoisomerase II DNA gyrase demonstrated significant interactions of these inhibitors with target protein, which provided valuable insights into their potent antibacterial activity. |
| Faculty |
Harminder Kaur
|
|
hkaur@pec.edu.in
|
|
| Collaborations | Govt. Home Science College, CSIR-Central Drug Research Institute Lucknow, Dr. B. R. Ambedkar National Institute of Technology Jalandhar |
| More Information | DOI: https://doi.org/10.1016/j.molstruc.2021.130924 |